In thermodynamics, chemical thermodynamics is the mathematical study of the interrelation of heat and work with chemical reactions or with a physical change of state within the confines of the laws of thermodynamics. Chemical thermodynamics can be generally thought of as the application of mathematical methods to the study of chemical questions and is concerned with the spontaneity of processes.
The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the "fundamental equations of Gibbs" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.
History
The primary objective of chemical thermodynamics is the establishment of a criterion for the determination of the feasibility or spontaneity of a given transformation. In this manner, chemical thermodynamics is typically used to predict the energy exchanges that occur in the following processes:
The following state functions are of primary concern in chemical thermodynamics:
Most identities in chemical thermodynaimcs arise from application of the first and second laws of thermodynamics, particularly the law of conservation of energy, to these state functions.
Chemical reactions
Phase changes
The formation of solutions
Internal energy (U)
Enthalpy (H).
Entropy (S)
Gibbs free energy (G)
 Overview
OverviewIt is the potential of a chemical substance to undergo a transformation through a chemical reaction or transform other chemical substances. Breaking or making of chemical bonds, involves energy, that may be either absorbed or evolved from a chemical system.
Energy that can be released (or absorbed) because of a reaction between a set of chemical substances is equal to the difference between the energy content of the products and the reactants.This change in energy is called the change in internal energy of a chemical reaction. It can be calculated using the formula
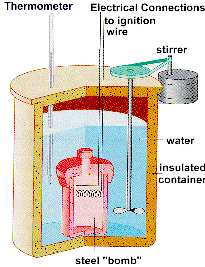
ΔUproducts is the internal energy of formation of the product molecules. The internal energy change of a process is equal to the heat change if it is measured under conditions of constant volume, as in a closed rigid container such as a bomb calorimeter. However, under conditions of constant pressure, as in reactions in vessels open to the atmosphere, the heat change measured is not always equal to the internal energy change, because pressure-volume work also releases or absorbs energy. (The heat change at constant pressure is called the enthalpy change, in this case the enthalpy of formation).
Another useful term is the heat of combustion, it is the energy released due to a combustion reaction and often applied in the study of fuels. Food is similar to hydrocarbon fuel and carbohydrate fuels, and when it is oxidized, its caloric content is similar (though not assessed in the same way as a hydrocarbon fuel-- see food energy).
In chemical thermodynamics the term used for the chemical potential energy is chemical potential and for chemical transformation an equation most often used is Gibbs-Duhem equation
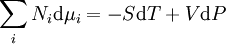
Chemical energy
In most cases of interest in chemical thermodynamics there are internal degrees of freedom and processes, such as chemical reactions and phase transitions, which always create entropy unless they are at equilibrium, or are maintained at a "running equilibrium" through "quasi-static" changes by being coupled to constraining devices, such as pistons or electrodes, to deliver and receive external work. Even for homogeneous "bulk" materials, the free energy functions depend on the composition, as do all the extensive thermodynamic potentials, including the internal energy. If the quantities { Ni }, the number of chemical species, are omitted from the formulae, it is impossible to describe compositional changes.
Gibbs function
No comments:
Post a Comment